

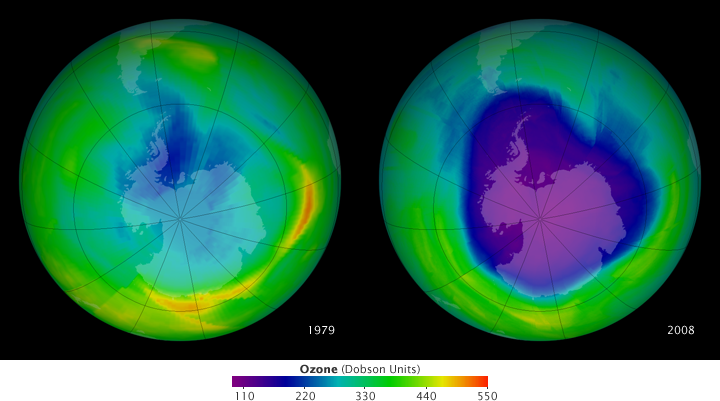
The stratospheric ozone layer protects life on Earth by absorbing ultraviolet light, which damages DNA in plants and animals (including humans) and leads to skin cancer. Prior to 1979, scientists had not observed concentrations below 220 Dobson Units. But in the early 1980s, through a combination of ground-based and satellite measurements, scientists began to realize that Earth’s natural sunscreen was thinning dramatically over the South Pole each spring. This large, thin spot in the ozone layer caused by chloroflourocarbons (CFCs) came to be known as the ozone hole.
This pair of images show the beginning and end of a nearly 30-year series of images that are part of our new World of Change: Antarctic Ozone Hole feature. The 1979 image was captured by NASA’s Total Ozone Mapping Spectrometer (TOMS) instrument aboard Nimbus-7, and the 2008 image is from the Royal Netherlands Meteorological Institute Ozone Monitoring Instrument (OMI) that flies on NASA’s Aura satellite. Purple and dark blue areas are part of the ozone hole.
As the images show, the word hole isn’t literal; no place is empty of ozone. Scientists use the word hole as a metaphor for the area in which ozone concentrations drop below the historical threshold of 220 Dobson Units. Using this metaphor, they can describe the hole’s size and depth. These maps show the state of the ozone hole each year on the day of maximum depth—the day the lowest ozone concentrations were measured.
In 1979, the ozone hole reached its maximum depth on September 30. At 194 Dobson Units (DU), it was not far below the historical low. The hole was confined to a relatively small area centered on the Antarctic Peninsula and the Weddell Sea to its east. Almost three decades later, the ozone concentration during the 2008 Southern Hemisphere spring bottomed out on October 4, 2008, at just 100 DU. The ozone hole encompassed virtually all of Antarctica and reached across the Southern Ocean toward the tip of South America.
The global recognition of CFCs’ destructive potential led to the 1989 Montreal Protocol banning the production of ozone-depleting chemicals. Scientists estimate that about 80 percent of the chlorine (and bromine, which has a similar ozone-depleting effect) in the stratosphere over Antarctica today is from human, not natural, sources. Models suggest that the concentration of chlorine and other ozone-depleting substances in the stratosphere will not return to pre-1980 levels until the middle decades of this century. These same models predict that the Antarctic ozone layer will recover around 2040. On the other hand, because of the impact of greenhouse gas warming, the ozone layer over the tropics and mid-southern latitudes may not recover for more than a century, and perhaps not ever.
NASA images courtesy Goddard Space Flight Center Ozone Processing Team.