|
What Determines How Much Ultraviolet Radiation Reaches the Earth’s
Surface?
The amount of UV radiation reaching the Earth’s surface varies widely
around the globe and through time. Several factors account for this
variation at any given location. They are discussed below in order of
importance, and descriptions of their effects appear in succeeding
paragraphs.

The effects of ultraviolet radiation
decrease with depth in the water column. (Image courtesy of NOAA)
Cloud Cover
Cloud cover plays a highly influential role in the amount of both UV-A
and UV-B radiation reaching the ground. Each water droplet in a cloud
scatters some incoming UV radiation back into space, so a thick cover of
clouds protects organisms and materials from almost all UV. The larger
the percentage of the sky that is covered by clouds, the less UV reaches
the ground. The more opaque the cloud, the less UV-B. However, thin or
broken cloud cover can be deceiving to people who are sunbathing, and
the result can be an unexpected and severe sunburn.
Ozone in the Stratosphere
Ozone is the combination of three oxygen atoms into a single molecule
(O3). It is a gas produced naturally in the stratosphere where it
strongly absorbs incoming UV radiation. But as stratospheric ozone
decreases, UV radiation is allowed to pass through, and exposure at the
Earth’s surface increases. Exposure to shorter wavelengths increases by
a larger percentage than exposure to longer wavelengths. Scientists can
accurately estimate the amount of UV-B radiation at the surface using
global data from satellites such as NASA’s TOMS (Total Ozone
Mapping Spectrometer), GOME (Global Ozone Monitoring Experiment) and Aura (will open in a
new window), to be launched in 2003, satellites. These satellite
measurements are compared to ground-based measurements to ensure that
the satellite data are valid. To calculate the reduction of UV-B by
ozone, scientists consider the total ozone in a column of air from the
stratosphere to the Earth’s surface. At mid-latitudes, a decrease of one
percent in ozone may result in an increase of between one (310 nm) and
three (305 nm) percent of potentially harmful UV-B at the surface during
mid-summer when UV-B is highest.
Ozone depletion is greater at higher latitudes, (toward the North and
South Poles) and negligible at lower latitudes (between 30 degrees N and
30 degrees S). This means that decreases in ozone over Toronto are
likely to be greater than those over Boston, and those over Boston
greater than those over Los Angeles, while Miami will typically see the
least ozone depletion of the four cities. However, cities at lower
latitudes generally receive more sunlight because they are nearer the
equator, so UV levels are higher even in the absence of ozone depletion.
If ozone were to decrease at lower latitudes, southern cities would
experience a greater absolute increase in UV-B than cities in the north
for the same amount of ozone depletion.
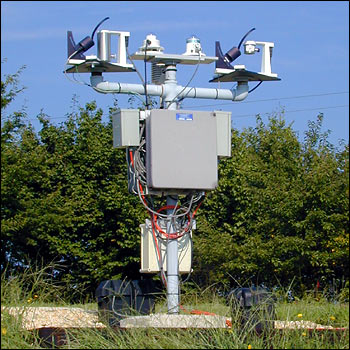
The U.S. Department of Agriculture maintains
an extensive network of radiometers to monitor ultraviolet B (UV-B)
radiation across the country. The one pictured above is in Beltsville, Maryland.
(Photograph by Jeannie Allen)
Oblique angle of sunlight reaching the surface
At any given time, sunlight strikes most of the Earth at an oblique
angle. In this way, the number of UV photons is spread over a wider
surface area, lowering the amount of incoming radiation at any given
spot, compared to its intensity when the sun is directly overhead. In
addition, the amount of atmosphere crossed by sunlight is greater at
oblique angles than when the sun is directly overhead. Thus, the light
travels through more ozone before reaching the Earth’s surface, thereby
increasing the amount of UV-B that is absorbed by molecules of ozone and
reducing UV-B exposure at the surface.
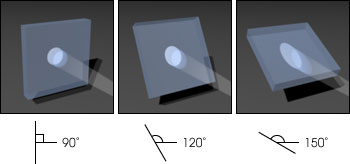
The three images above illustrate how a change in angle
between the sun and the Earth’s surface affect the intensity of sunlight (and UV-B)
on the surface. When the sun is directly overhead, forming a 90° angle with the surface,
sunlight is spread over the minimum area. Also, the light only has to pass through the atmosphere
directly above the surface. An increased angle between the sun and the surface—due to
latitude, time of day, and season—spreads the same amount of energy over a wider area,
and the sunlight passes through more atmosphere, diffusing the light. Therefore, UV-B radiation
is stronger at the equator than the poles, stronger at noon than evening, and stronger in
summer than winter. (Illustration by Robert Simmon)
Aerosols
Unlike clouds, aerosols in the
troposphere, such as dust and smoke, not only scatter but also absorb
UV-B radiation. Usually the UV reduction by aerosols is only a few
percent, but in regions of heavy smoke or dust, aerosol particles can
absorb more than 50 percent of the radiation.
While the presence of aerosols anywhere in the atmosphere will always
scatter some UV radiation back to space, in some circumstances, aerosols
can contribute to an increase in UV exposure at the surface. For
example, over Antarctica, cold temperatures cause ice particles (Polar
Stratospheric Clouds) to form in the stratosphere. The nuclei for these
particles are thought to be sulfuric acid aerosol, possibly of volcanic
origin. The ice particles provide the surfaces that allow complex
chemical reactions to take place in a manner than can deplete
stratospheric ozone.

The eruption of Mt. Pinatubo in
1991 injected sulfate aerosols into the stratosphere, significantly
though temporarily depleting stratospheric ozone and resulting in an
increase of UV-B reaching the Earth’s surface. Over millions of years,
the biosphere has evolved to deal with temporary increases in UV from
reductions in stratospheric ozone by natural causes such as volcanic
eruptions, but has not had the time required to adjust to long-term
ozone reductions attributed to human activities of the last 30 years.
(Photograph courtesy USGS)
Water Depth
UV-B exposure decreases rapidly at increasing depths in the water
column. In other words, water and the impurities in it strongly absorb
and scatter incoming UV-B radiation. Some substances that are dissolved
in water, such as organic carbon from nearby land, will also absorb UV-B
radiation and enhance protection of microorganisms, plants, and animals
from UV-B. Different masses of water at different locations contain
different amounts of such dissolved substances and other particles,
making evaluation of UV damage very difficult.
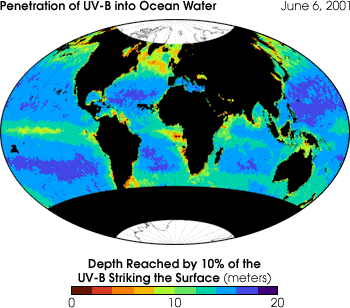
Ultraviolet B
(UV-B) radiation reaches different depths in ocean water depending on water
chemistry, the density of phytoplankton, and the presence of sediment and other particulates.
The map above indicates the average depth UV-B penetrates into ocean water. At the depth
indicated, only 10 percent of the UV-B radiation that was present at the water’s
surface remains. The rest was absorbed or scattered back towards the ocean surface.
(Image courtesy Vasilkov et al., JGR-Oceans, 2001)
Elevation
Living organisms at high elevations are generally exposed to more solar
radiation and with it, more UV-B than organisms at low elevations. This
is because at high elevations UV-B radiation travels through less
atmosphere before it reaches the ground, and so it has fewer chances of
encountering radiation-absorbing aerosols or chemical substances (such
as ozone and sulfur dioxide) than it does at lower elevations.

Ecosystems at high altitudes, such as this lake in the
Rocky Mountains of Colorado, receive more exposure to ultraviolet
radiation than ecosystems at low altitudes. (Photo courtesy Philip
Greenspun © 1994)
Reflectivity of the Earth’s Surface
As a highly reflective substance, snow dramatically increases UV-B
exposure near the Earth’s surface as it reflects most of the radiation
back into the atmosphere, where it is then scattered back toward the
surface by aerosols and air molecules. Fresh snow can reflect much as
94 percent of the incoming UV radiation. In contrast, snow-free lands
typically reflect only 2-4 percent of UV and ocean surfaces reflect
about 5-8 percent (Herman and Celarier 1997).
next: How Much Are We
Getting?
back: Effects on the
Biosphere |
|

Ultraviolet Radiation
Introduction
Effects on the Biosphere
What Determines UV at the Surface?
How Much Are We Getting?
Predictions and Monitoring
References
|

