

Radiation |
Unless it has a temperature of absolute zero (-273°C) an object reflects, absorbs, and emits energy in a unique way, and at all times. This energy, called electromagnetic radiation, is emitted in waves that are able to transmit energy from one place to another. For example, this computer, trees, air, the Sun, the Earth, and all the stars and planets are reflecting and emitting a wide range of electromagnetic waves. These waves originate from billions of vibrating electrons, atoms, and molecules, which emit and absorb electromagnetic radiation in unique combinations of wavelengths.
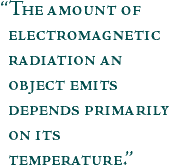
Objects emit more intense radiation at higher frequencies as they are heated. A burned-out lightbulb [at room temperature, 27°C (300 Kelvin)] emits no visible radiation, while a heat lamp [677°C(950 Kelvin)] emits most of its energy in the thermal infrared and a little low frequency (red) light. An incandescent bulb [2223°C (2500 Kelvin)] gives off orange-yellow light, although only ten percent of its emitted energy is visible light—the rest is heat. (Images by Robert Simmon) The amount of electromagnetic radiation an object emits depends
primarily on its temperature. The higher the temperature of an object,
the faster its electrons vibrate and the shorter its peak wavelength of
emitted radiation. Conversely, the lower the temperature of an object,
the slower its electrons vibrate, and the longer its peak wavelength of
emitted radiation. This concept can be shown by gripping the end of a
long rope and shaking it. Rapidly shaking the rope (high temperature)
results in a series of short waves travelling along it, while shaking it
slowly (low temperature) results in a series of longer waves. |
 Remote Sensing
Related Data Sets: |
 |
next: Electromagnetic Spectrum |