| |
Step 1. Sunlight splits ozone into an oxygen molecule and an oxygen atom.

An electronically excited oxygen atom reacts with water vapor to generate hydroxyl. (Oxygen and water molecules are abundant in the atmosphere, and so are available to take part in ozone-forming reactions.)

Step 2. Hydroxyl reacts readily with other chemicals, and initiates another sequence of reactions. It combines with methane, producing water and a chemical called a methyl radical.

Step 3. The methyl radical combines with oxygen to produce methyl peroxy radical.

Step 4. The methyl peroxy radical combines with nitric oxide (from fossil fuel combustion) to produce a methyloxy radical and nitrogen dioxide.
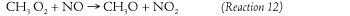
Step 5. Sunlight splits the nitrogen dioxide into nitric oxide and atomic oxygen, which combines with molecular oxygen to yield ozone, as in Reactions 5 and 6 above.


Some of the methyl oxy radical from Step 4 can participate in a different series of reactions that ultimately also make more nitrogen dioxide available for ozone formation. In this second series, the methyl oxy radical combines with oxygen to produce formaldehyde and a hyperoxy radical.
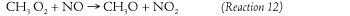
The hyperoxy radical reacts with nitric oxide to yield hydroxyl and nitrogen dioxide.

Sunlight splits nitrogen dioxide into nitric oxide and atomic oxygen, which combines with molecular oxygen to yield ozone, as in Reactions 5 and 6 above (copied below).


A third series of reactions, one involving formaldehyde produced in Reaction 12, can ultimately produce even more nitrogen dioxide that then becomes available for ozone formation. Sunlight splits formaldehyde into a formyl radical and atomic (single atom) hydrogen. Both of these species are extremely short lived and react almost instantaneously with molecular oxygen to form hyperoxy radicals.
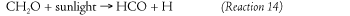


The hyperoxy radical and nitric oxide then combine to form hydroxyl and nitrogen dioxide.

Reactions 5 and 6 then take place, resulting in more ozone.
Some Chemicals Involved in Ozone Formation:
| CH2O | formaldehyde |
| CH3 | methyl radical |
| CH3O | methyloxy radical |
| CH3 O2 | methyl peroxy radical |
| CH4 | methane |
| CO | carbon monoxide |
| H | hydrogen |
| HCO | formyl radical |
| HO2 | hyperoxy radical |
| H2O | water |
| NO | nitric oxide |
| NO2 | nitrogen dioxide |
| NOx | nitrogen oxides (NO & NO2) |
| N2O | nitrous oxide |
| OH | hydroxyl radical |
| O | atomic oxygen |
| O2 | molecular oxygen |
| O3 | ozone |
back: Chemistry of Ozone Formation |
|
|

