

A Titanium “Thermometer” |
|||
Watson’s own study of the Australian zircons supports the idea of a cooler, wetter Hadean. Several years ago, Watson and the students working in his lab began work on a titanium “thermometer”—an equation that describes how the titanium concentration in zircons changes as their crystallization temperature changes. Once they had the equation, geologists could estimate the temperature at which the Australian zircons crystallized more than 4 billion years ago by measuring the amount of titanium they contain. |
|||
To develop the “thermometer” equation, Watson and his team grew synthetic zircons in the presence of titanium at controlled temperatures in his lab. Then they measured how much titanium appeared in crystals grown at different temperatures. They also analyzed titanium concentrations in naturally occurring zircons found at several locations across the globe, whose crystallization temperature had been determined through independent tests by other scientists. |
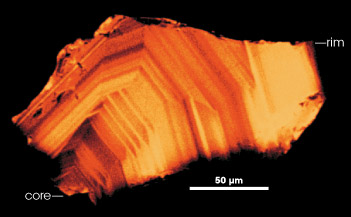
This small Jack Hills zircon (50 micrometers is about the width of a human hair) crystallized slowly, leaving concentric bands. The crystal grew from the core (left) to the rim (right). As crystals form, they incorporate chemicals from their surroundings. The titanium concentration in the zircon reveals the temperature at which the crystal formed. (Image courtesy Bruce Watson, Rensselaer) | ||
 |
“I knew all along that by far the most compelling application of the thermometer would be to the [Jack Hills] zircons, but people don’t part with them too willingly,” explained Watson. To gain access to the zircons, Watson teamed up with an old friend, Mark Harrison of the Australian National University, whose department had made studying the zircons a top priority. “He had access, I had the tool, and so we pooled our resources,” explained Watson. When Watson compared the titanium concentration of the Jack Hills zircons to his thermometer, he discovered something interesting. All the zircons appeared to have formed within a very restricted temperature range: about 680 plus or minus 20 degrees Celsius. |
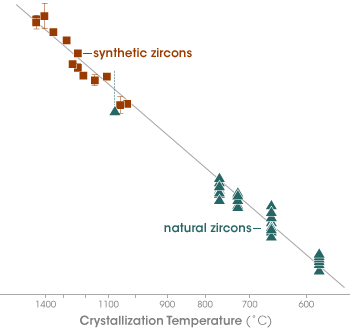
Geologists call a mathematical equation that describes how much of a particular chemical a mineral will contain when it forms at a given temperature a “thermometer.” Watson and his team developed a thermometer for the amount of titanium in zircon crystals by growing some zircons at fixed temperatures in their lab (reddish-brown data points) and by analyzing natural zircons (green points) whose formation temperatures were already known. By comparing the titanium concentration of the Jack Hills zircons to the thermometer, the team determined the temperature at which the crystals formed. (Graph adapted from Watson and Harrison 2005) | |
 |
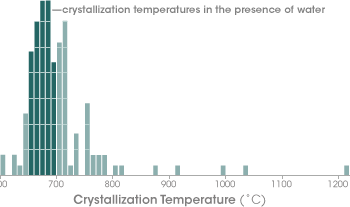
To a geologist, that is a very special temperature range, explains Watson. “Any rock heated in the presence of water—any rock, at any time, in any circumstance—will begin to melt at between 650 and 700 degrees. This is the only terrestrial process that occurs so predictably,” said Watson. Watson thinks the most likely scenario is that the Jack Hills zircons formed in a continental crust environment similar to what we know today, in which water-saturated rocks melt as they are subducted along plate boundaries or are buried in deep sediment. “Some critics have said the water could have been coming up into the zircon-forming area from down in the mantle [the layer of Earth beneath the crust], and our results don’t directly contradict that explanation,” acknowledged Watson. “But taken with previously published zircon evidence such as the oxygen isotope data, we feel our results point more strongly toward the idea of surface water.” |
The titanium thermometer revealed that the majority of Jack Hills zircons crystallized at temperatures between 660 and 700° Celsius. This temperature range corresponds to the low end of the temperature range at which any rock heated in the presence of water will begin to melt. (Graph adapted from Watson and Harrison 2006) |