

|
|
Is the stratospheric ozone layer recovering?The stratospheric ozone layer shields life on Earth from harmful ultraviolet (UV) solar radiation (wavelengths shorter than 340 nm). Research shows that excess exposure to the Sun’s UV radiation is harmful to plants, including crops, and causes skin cancer and eye problems in humans and animals. Excess UV radiation exposure may also suppress the human immune system. Ozone forms naturally in the stratosphere as incoming solar UV radiation hits oxygen molecules (O2) and breaks them apart into free-floating individual oxygen atoms. These oxygen atoms can then combine with O2 molecules to form ozone molecules (O3). Ozone is destroyed when an ozone molecule combines with an oxygen atom to form two oxygen molecules; or through certain chemical reactions involving molecules containing hydrogen, nitrogen, chlorine, or bromine atoms. (For more details on how stratospheric ozone is formed and destroyed, please see NASA’s Ozone fact sheet. The atmosphere maintains a natural balance between ozone formation and destruction. But the natural balance of chemicals in the stratosphere has changed over the last three decades, particularly due to the presence of man-made chlorofluorocarbons (CFCs). CFCs are produced by chemical industries for use as refrigerants, solvents, and propellants. Because CFCs are non-reactive they tend to build up in the atmosphere and are eventually destroyed high in the stratosphere where they are no longer shielded by the ozone layer from UV radiation. Destruction of CFC molecules yields free-floating chlorine atoms, which play an active role in destroying ozone molecules. Other man-made gases, such as nitrous oxide (N2O) and bromine compounds, are also broken down in the stratosphere and play active roles in ozone destruction. |
|
 |
|
|
Satellite observations of the ozone layer began in the 1970s when the possibility of ozone depletion was just becoming an environmental concern. NASA’s Total Ozone Mapping Spectrometer (TOMS) and Stratospheric Aerosol and Gas Experiment (SAGE) have provided long-term records of ozone. In 1985 the British Antarctic Survey reported an unexpectedly deep ozone depletion over Antarctica. The annual occurrence of this depletion, popularly known as the ozone hole, alarmed scientists. Specially equipped high-altitude NASA aircraft established that the ozone hole was due to man-made chlorine. TOMS and SAGE data also showed smaller but significant ozone losses outside the Antarctic region. In 1987 an international agreement known as the Montreal Protocol restricted CFC production. In 1992, the Copenhagen amendments to the Montreal Protocol set a schedule to eliminate all production of CFCs. |
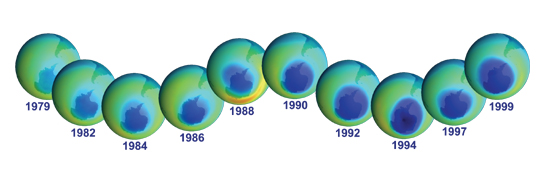 These TOMS images illustrate the development of the ozone hole during the 1980s and 1990s. Dark blue colors correspond to the thinnest ozone, while light blue, green, and yellow pixels indicate progressively thicker ozone. (Image courtesy of the NASA GSFC Scientific Visualization Studio.) |

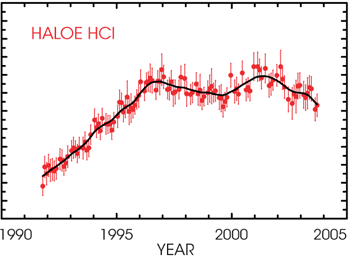
Ground-based data have shown that CFC amounts in the troposphere are leveling off, while data from the Halogen Occultation Experiment (HALOE) on the Upper Atmosphere Research Satellite (UARS) show that amounts of HCl, a chlorine reservoir that is produced when CFCs are broken apart, are leveling off as well (See Global HCl figure). Recent studies show that the rate of ozone depletion is also decreasing. |
Thin clouds made of ice, nitric acid, and sulfuric acid mixtures form in the polar stratosphere when temperatures drop below -88°C (-126°F). In such polar stratospheric clouds (PSCs) active forms of chlorine are released from their reservoirs. This particular PSC appeared over Iceland at an altitude of 22 km on February 4, 2003. Its beautiful colors result from diffraction of sunlight by ice particles. (Photo courtesy Mark Schoeberl, NASA GSFC). |
||
 |
Recovery of the ozone layer may not be as simple as eliminating the manufacture of CFCs. Climate change will alter ozone recovery because greenhouse gas increases will cause the stratosphere to cool. This cooling may temporarily slow the recovery of the ozone layer in the polar regions, but will accelerate ozone recovery at low and middle latitudes. What will Aura do?Aura’s instruments will observe the important sources, radicals, and reservoir gases active in ozone chemistry. Aura data will improve our ability to predict ozone change. Aura data will also help untangle the roles of transport and chemistry in determining ozone trends. For details about how Aura's individual instruments contribute to understanding stratospheric ozone, please see the "Aura's Instruments" section of this Fact Sheet. next: What are the processes controlling air quality? |
UARS HALOE measurements of stratospheric chlorine (HCl) at 55 km show that international controls on CFCs are working. HCl amounts have leveled off and are now decreasing. Aura's MLS will continue the HALOE HCl record. |