

It doesn’t take a lot of technology to see that the ocean is blue. And when it comes to the blueness of the ocean, it doesn’t get much more blue than where I am. I’m sitting on the research vessel Nathaniel B. Palmer—the largest icebreaker that supports the United States Antarctic Program—on an oceanographic expedition across the South Pacific Ocean, my current home, office, and laboratory. On this voyage, however, the Palmer has broken no ice.
Our Global Ocean Ship-based Hydrographic Investigations Program (GO-SHIP) P06 campaign departed Sydney, Australia, on July 3, successfully ending the first leg of this journey on August 16 in Papeete, French Polynesia (Tahiti). This is where our team from NASA Goddard Space Flight Center (Scott Freeman, Michael Novak, and I) joined dozens of other scientists, graduate students, marine technicians, officers and crew members, for the second and final leg that will end in the port of Valparaiso, Chile, on September 30. The GO-SHIP program is part of the long history of international programs that have criss-crossed the major ocean basins, gathering fundamental hydrographic data that support our ever-growing understanding of the global ocean and its role in regulating the Earth’s climate, and of the physical and chemical processes that determine the distribution and abundance of marine life. This latter topic regarding the ecology of the ocean is what brings our Goddard team along for the ride.
The P06 ship track, for the most part, follows along 32.5° of latitude south. That route places our course just south of the center of the South Pacific Gyre—the largest of the five major oceanic gyres, which form part the global system of ocean circulation. The Gyre—on average—holds the clearest, bluest ocean waters of any other ocean basin. This blueness is the macroscopic expression of its dearth of ocean life. We have seen nary a fish nor other ships since we departed Tahiti—this is not a major shipping route. Oceanic gyres are often called the deserts of the sea. On land, desert landscapes are limited in their capacity to support life by the availability of water. Here, lack of water is not the issue. Water, however, is at least the co-conspirator in keeping life from flourishing. Physics, as it turns out, is what holds the key to this barren waterscape.
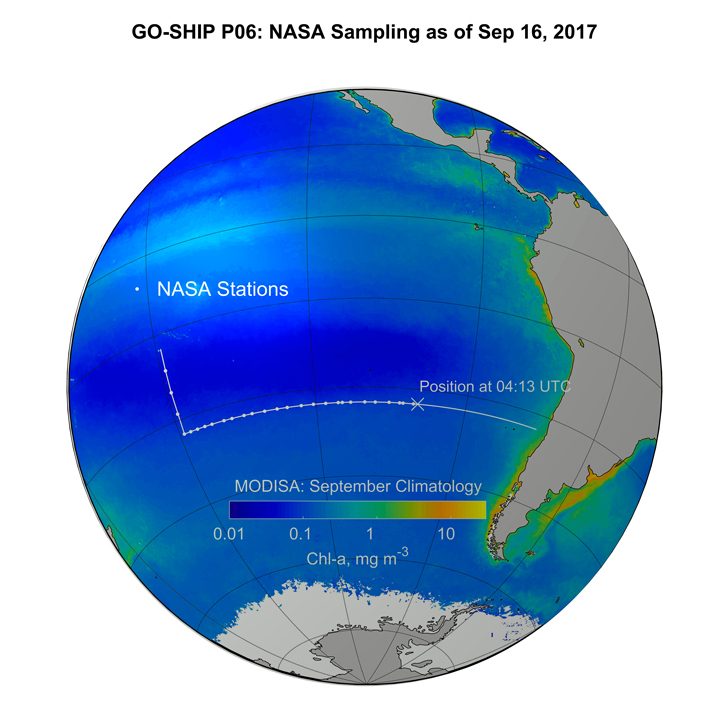
This map (above) shows the MODIS chlorophyll climatology with the ship track superimposed.
Due to the physics of fluids on a rotating sphere, such as our planet, the upper ocean currents slowly rotate counterclockwise around the edges of the center of the Gyre–as a proper Southern Hemisphere gyre should—and a fraction of that flow is deflected inward, towards its center. With water flowing towards the center from all directions, literally piling up and bulging the surface of the ocean–albeit, by just a few centimeters across thousands of miles–gravity pushes down on this pile of water. This relentless downward push puts a lock on life. The pioneers of life in the ocean, the tiny microscopic plants, known as phytoplankton, which drift in the currents, and grow on a steady mineral diet of carbon dioxide, nitrogen and phosphorus, and a dash of iron—and expel oxygen gas as a by-product, to the great benefit of life on Earth—must obtain most of their material sustenance from the ocean below. Layers of denser water trap the nitrogen and phosphorus-rich water at depth, keeping it too far down, where not enough light can reach it to spark the engine of photosynthesis that allows plants to grow.
Why are we here and where does NASA come into this story? Since the late 1970s, NASA has pursued—experimentally at first, and now as a sustained program—measuring the color of the oceans from Earth-orbiting satellites as a means to quantify the abundance of microscopic plant life. Microbiology from space, in a way. Formally, though, we call it “ocean color remote sensing.” Whizzing by at altitudes of several hundred miles, atop of the atmosphere, bound to polar orbits that allow satellites to scan the entire surface of the globe every couple of days, carrying instrument payloads of meticulously engineered spectro-radiometers—cameras capable of measuring the quantity and quality, or color, of the light that reaches its sensors. This is where our work aboard the R/V Palmer comes into the story. The data the satellites beam down from orbit do not directly measure how much plant life there is in the ocean. Satellite instruments give us digital signals that relate to the amount of light that reached their sensors. It is up to us to translate—to calibrate—those signals into meaningful, and accurate, measurements of plant life—or temperature, salinity, sediment load, sea level height, wind and sea surface roughness, or any other of the many environmental or geophysical variables satellite sensors can help us detect at the surface of the ocean. To properly calibrate a satellite sensor and validate its data products, we must obtain field measurements of the highest possible quality. That is what our team from NASA Goddard is here to do.
Around midday, typically the time of the ocean color satellite flying over our location, we perform our measurements and collect samples. We measure the optical properties of the water with our instruments to compare what we see from the R/V Palmer to what the satellites measure from their orbit above Earth’s atmosphere. At the same time that we perform our battery of optical measurements, we also collect phytoplankton samples to estimate their abundance, species composition as well as the concentration of chlorophyll-a, the green pigment common to most photosynthesizing organisms like plants, including phytoplankton. By collecting these two types of measurements at once, light and microscopic plant abundance, we are able to build the mathematical relationships that make the validation of the satellite data products possible.

The optical instrument being lowered into the South Pacific Ocean. Credit: Lena Schulze/FSU

Scott Freeman of NASA works with a R/V Nathaniel Palmer crew member to prepare an instrument for deployment over the side of the ship to collect opHcal measurements. Credit: Lena Schulze/FSU

Mike Novack of NASA studies the optical and biological characteristics of seawater samples in the ship’s laboratory. Credit: Joaquin Chavez/NASA
The waters of the South Pacific Gyre are an ideal location for gathering validation quality data, perhaps one the most desirable to do so, because there are few complicating factors and sources of uncertainty that blur the connection we want to establish between the color of the water and phytoplankton life abundance. Our measurements will extend NASA’s ocean chlorophyll-a dataset to some of the lowest values on Earth. The water here is blue; in fact, it’s the bluest ocean water on Earth.
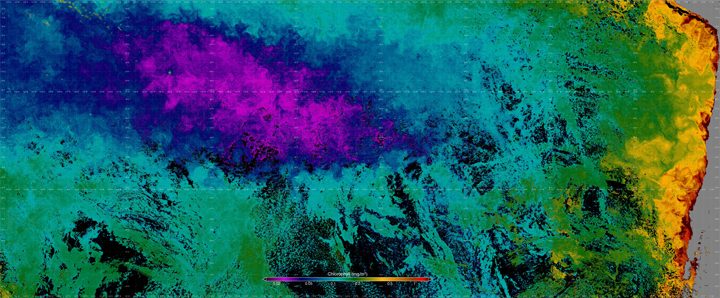
This false-color image is a composite, assembled from data acquired by the the Visible Infrared Imaging Radiometer Suite (VIIRS) on the Suomi NPP satellite between September 10-17, 2017. Yellow represents areas with the highest chlorophyll concentrations and purples are the lowest. Credit: Norman Kuring/NASA
We have spent the last few weeks discussing the differences between inherent and apparent optical properties in the ocean and how we measure them. Now let’s take a moment to appreciate the information these data give us. I am sure many of you have seen satellite images of the ocean, hurricanes, etc. on the news and at other outlets. A lot of work goes into each and every one of those images and they can show remarkable things on a global scale that would be difficult to detect through fieldwork alone.
Below you will see a true color image of a phytoplankton bloom in the Barents Sea that was acquired on August 24, 2012 by NASA’s MODIS Aqua satellite . The Barents Sea is part of the Arctic Ocean and is located off the northern coasts of Norway and Russia. The word phytoplankton is Latin for plant (phyto) and to wander or drift (plankton). Phytoplankton can photosynthesize and produce energy from sunlight just like plants do on land. They also take up carbon dioxide and release oxygen like land plants. Phytoplankton are an important component of the food chain as other organisms such as zooplankton and fish use them as a food source. Additionally, they play an important role in the global carbon budget because they can use the carbon (CO2) that is absorbed by the ocean to make sugars.
There are many types of phytoplankton that bloom in the ocean including diatoms, dinoflagellates, and coccolithophores, just to name a few. You can learn more about different phytoplankton groups here. They all rely on specific nutrients, such as nitrogen (NO3, NO2, etc.), phosphorous (PO4, etc.) and trace metals (iron, magnesium, etc.). Phytoplankton blooms occur when many individuals, or cells, of one species are present in one region of a body of water (lake, estuary, open ocean), altering the color of the water. In the image above, the water is dominated by a species of coccolithophore. Coccolithophores are a type of phytoplankton that are covered with plates made of calcite called coccoliths. Calcite, or calcium carbonate, is white giving that creamy white look to the water. You can learn more about this coccolithophore bloom here.
This next image was recently featured on NASA’s OceanColor website. It was taken off the western coast of Africa where a current called the Benguela current flows northward bringing water from the South Atlantic and Indian Ocean. In this image from April 10, 2014 we can see a yellow discoloration in the water. I just learned that this area is prone to toxic hydrogen sulfide precipitation events that have been known to cause fish and other marine animal mortalities. Amazingly, these events have been captured using satellite imagery. However, the cause of this particular discoloration has not, as of this post, been determined. This is where fieldwork comes in! Still, it is very cool! Thanks to Norman Kuring at NASA Goddard for locating this feature.
You can enjoy many other satellite images at NASA’s Visible Earth website.
References:
http://www.worldatlas.com/aatlas/infopage/barentssea.htm
http://earthobservatory.nasa.gov/Features/Phytoplankton/
http://www.bigelow.org/foodweb/microbe0.html
http://protozoa.uga.edu/portal/coccolithophores.html
http://oceancolor.gsfc.nasa.gov/
http://oceancurrents.rsmas.miami.edu/atlantic/benguela.html
http://oceancolor.gsfc.nasa.gov/cgi/image_archive.cgi?i=423
http://www.sciencedirect.com/science/article/pii/S0967063703001754
ACKNOWLEDGEMENTS: NASA’s Ocean Ecology Laboratory Field Support Group is participating in the US Repeat Hydrography, P16S field campaign under the auspices of the International Global Ocean Ship-Based Hydrographic Investigations Program (GO-SHIP). The US Climate Variability and Predictability Program (CLIVAR), NOAA and the NSF sponsor this campaign.
One of the greatest tools used by oceanographers today for measuring ocean processes is the CTD. CTD stands for Conductivity, Temperature and Depth. Conductivity is a measure of ocean salinity. The CTD is used to collect profile data in the ocean. The CTD is typically accompanied by a carousel, or rosette, of large bottles (Niskins) that can hold about 10 liters (2.6 U.S. gallons) of water. Some Niskins are large enough to hold 30 liters. These bottles have spring-loaded caps that can be triggered to close at specified depths. The CTD and other sensors, such as a chlorophyll fluorometer, and an Acoustic Doppler Current Profiler (ADCP) that measures current velocities, and other sensors can also be attached within the rosette package.
The whole package is connected to a very long cable and is mechanically lowered by a winch operator down through the water column, which is called the ‘downcast.’ During the downcast, information about salinity, temperature, depth and data from the other sensors are sent to a computer on board the ship. The computer is connected through the cable that is lowering the package. The downcast is halted once the package reaches close to the ocean floor. When the CTD is raised back to the surface, the ‘upcast’, each of the Niskin bottles is closed at assigned depths, collecting water as it travels back to the surface.
Once the Rosette package is back aboard the ship, the scientists are able to collect water from the bottles for their analyses. The parameters collected and analyzed during CLIVAR campaigns includes but are not limited to: salinity, oxygen, nutrients, chlorofluorocarbons (CFCs), dissolved inorganic carbon (DIC), total alkalinity, pH, dissolved organic carbon (DOC), helium, and tritium.
Certain compounds, such as some radionuclide (tritium, carbon-14, etc.) and CFCs, can be used as ‘tracers’. These tracers are used to follow ocean currents and calculate the age of water parcels. CFCs were prominently used in refrigerators and air conditioning units until the 1970s when they were banned over the concern of ozone depletion. You can learn more about the CFC tracer program here. Through the WOCE and CLIVAR programs CFC concentrations have been measured all over the world.
The measurement of pH, total alkalinity and DIC are important for monitoring ocean acidification. Ocean acidification (OA) is the decrease in ocean pH as a result of an increase in carbon dioxide (CO2) absorption by seawater. OA is a prominent concern in today’s world. CO2 is pumped into the atmosphere from everyday human activities, such as emissions from vehicles and industrial pollution. Each year approximately 25% of the CO2 pumped into the atmosphere is absorbed by the ocean. Although plants can use CO2 for photosynthesis, the increase also has negative implications. As the amount of CO2 absorbed by the increases, the pH is expected to continue decreasing.
The pH of the ocean directly affects organisms that form calcium carbonate shells or structures, like corals, oysters, clams and sea urchins. An acidic environment causes the calcium carbonate to dissolve and makes it more difficult for the organisms to make their calcium carbonate skeletons. Therefore, it is important that programs like CLIVAR are monitoring global CO2 concentrations (part of the DIC pool), total alkalinity (the ability for the ocean to neutralize acids) and pH. We know that decreases in ocean pH can negatively impact marine organisms. You can see more about the effect of ocean acidification or marine organisms here.
As I was conducting some research for this blog post I came across this article that was posted at the Earth Observatory in 2008 about the global carbon budget. I thought it was appropriate to bring it back here.
References:
http://www.whoi.edu/home/oceanus_images/ries/calcification.html
http://www.pmel.noaa.gov/co2/story/What+is+Ocean+Acidification%3F
http://water.me.vccs.edu/exam_prep/alkalinity.html
http://earthobservatory.nasa.gov/Features/OceanCarbon/
http://rspb.royalsocietypublishing.org/content/275/1644/1767.full
ACKNOWLEDGEMENTS: NASA’s Ocean Ecology Laboratory Field Support Group is participating in the US Repeat Hydrography, P16S field campaign under the auspices of the International Global Ocean Ship-Based Hydrographic Investigations Program (GO-SHIP). The US Climate Variability and Predictability Program (CLIVAR), NOAA and the NSF sponsor this campaign.
The NASA FSG folks and the rest of the science crew on the US Repeat Hydrography P16S field campaign have experienced some weather delays, which can be expected when working the Southern Ocean close in time to the Southern Hemisphere winter. They encountered 45-knot winds (~51 miles per hour) sitting on top of their planned sampling area at around 60 degrees South latitude. For the safety of all they could not conduct science under those conditions. So they traveled ~200 miles north to escape the weather and then returned southward once the weather had settled. Below you will see some freeze frame clips from the movie Joaquin Chaves captured showing the large swells and the dramatic gray skies of the Southern Ocean. The movie was recorded through the porthole, safe inside the ship.
In spite of the rough weather, the FSG fellows have taken advantage of some calmer days to deploy a radiometer. A radiometer measures apparent optical properties or AOPs. You might recall from Blog #2 that we discussed Inherent Optical Properties or IOPS that characterize the absorption and scattering (reflecting) of light by dissolved and particulate materials in the water. The measurement of IOPs can be done at any time day or night. AOPs on the other hand must be measured during the daytime, preferable when there are clear skies and the sun is directly overhead. AOPs describe how the light is entering and exiting the water column. Remember that sunlight contains a whole spectrum of colors that are determined by their wavelength. We see what is called the “visible spectrum” like what is in the image below.
As the sunlight penetrates the water column, some of the light is absorbed, some is scattered (reflected) backward toward the sky and the rest is scattered forward into the depths of the ocean. Water itself absorbs most of the red light, so we never really see a red ocean (unless there is something unusual in the water).
The character of the light that is reflected back out of the water can be different than what went in. More specifically, the wavelengths or colors that are reflected back out are the colors that were not absorbed or scattered forward. Think about when you are at the beach, say Ocean City, MD or Rehoboth Beach, Delaware. The water typically looks brown or green, right? This is because particles, including both algae and sediment (and water), are absorbing all of the blue and red light, and the leftover colors of brown and green are reflected back out. Conversely, for those of you who have seen blue water in places like the Caribbean or around Bermuda, the circumstances are different. There are very few particles or phytoplankton in the water to absorb the blue light like in the coastal water. Therefore, that blue color is reflected back out , which is why the water looks so blue and pretty. A radiometer that the FSG is deploying on this field campaign measures the colors and amount of the light entering and exiting the water column. The radiometer is hand deployed and, I can tell you, it’s a lot of work!
Satellite instruments, such as SeaWiFS (no longer operational) and MODIS-Aqua (operational) measures this light just like the radiometer so that we can get beautiful images that you can see below.
Our FSG fellas are working very hard during this field campaign. But every now and then you need to have a little fun. Mike Novak sent this picture of a snowman that was built on the bow of the ship. I think the eyes, nose, and buttons are raisins.
References:
http://ushydro.ucsd.edu/p16s-weekly-reports/
http://www.oceanopticsbook.info/
http://oceancolor.gsfc.nasa.gov
ACKNOWLEDGEMENTS: NASA’s Ocean Ecology Laboratory Field Support Group is participating in the US Repeat Hydrography, P16S field campaign under the auspices of the International Global Ocean Ship-Based Hydrographic Investigations Program (GO-SHIP). The US Climate Variability and Predictability Program (CLIVAR), NOAA and the NSF sponsor this campaign.
The guys are finally on their way! The R/V Nathaniel B. Palmer set sail from Hobart, Tasmania on March 20, 2014 ( GMT +11 hours). The science party is made up of a total 29 scientists, 9 of which are graduate students. The first Go-SHIP station is located at 67°S, 150°W. While in transit, scientists will deploy the first Bio-Argo float of the campaign, 6 days from sail. An Argo float is a battery-operated, autonomous float that can move up and down the water column collecting temperature and salinity profiles up to a 2000m depth by pumping fluid into and out of a bladder to manipulate buoyancy. A Bio-Argo can collect measurements of chlorophyll-a and backscattering, in addition to salinity and temperature profiles. The deployment of Bio-Argo floats is particularly important for validating ocean color remote sensing data. For more information about Argo floats, you can proceed to the following links:
Setting up a scientific laboratory on a ship is no easy task. Space is usually limited and you must be able to play well with others. We have filtration equipment (the large wooden frames) set up to collect the biogeochemical parameters, i.e. phytoplankton pigments, particulate organic carbon and particle absorption. The parameters are collected onto small paper filters and frozen for future analyses back at NASA Goddard. We also have two instruments set up on board to measure colored dissolved organic matter (CDOM), which is like tea, compounds extracted from plant material that can flow out into the ocean via rivers. While in transit to the first station Mike, Joaquin and Scott are busy collecting samples.
A major addition to this year’s field campaign is a ‘souped-up’ underway-sampling system built by none other than Scott Freeman, our optics expert on board the Palmer. The set-up contains multiple instruments that collect dissolved and particulate absorption, CDOM fluorometry, chlorophyll and particle scattering at 660nm. The system is connected to the ship’s seawater system that pumps clean seawater from <10m depth through the ship and then to faucets at which the water can be accessed. The term ‘clean’ means the plumbing that facilitates seawater pumping to the laboratories is routinely checked for clogs and algae growth.
Lastly, a blog post isn’t complete without a gratuitous photo of macrofauna. Here is a photo of a petrel taken by Joaquin Chaves. Can anyone identify what kind of petrel this is?