

Ozone is Earth’s natural sunscreen, absorbing most of the incoming ultraviolet (UV) radiation from the sun and protecting life from DNA-damaging radiation. Chlorofluorocarbons (CFCs for short)—invented in the early 1890s and first used in the 1930s as refrigerants and propellants for chemical sprays—are ozone destroyers.
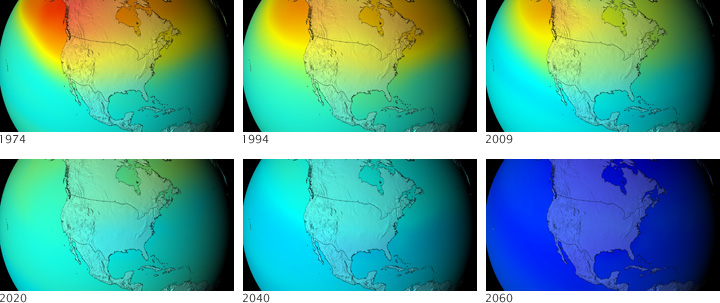
Earlier this year, a team of NASA-led scientists set out to predict what the ozone layer would have looked like today and in the future if countries around the world had not signed the Montreal Protocol Treaty banning ozone-depleting chemicals. This series of images shows ozone concentrations over the mid-latitudes of the Western Hemisphere, based on months of calculations by the Goddard Earth Observing System Chemistry-Climate Model.
The series of images starts with 1974, before CFCs had begun to do significant damage to the ozone layer. Concentrations of ozone in the stratosphere over the United States and Canada are high. By 1994, the model predicts that ozone concentrations over the region have fallen from highs above 500 Dobson Units to about 400. By the simulated year 2009, the ozone layer over much of the United States has thinned to only 300 Dobson Units.
By 2020, the model predicts that an ozone “hole”—concentrations below 220 Dobson Units—forms over the Arctic as well as the Antarctic. By 2040, the ozone hole is global. The UV index in mid-latitude cities reaches 15 around noon on a clear summer day (10 is considered extreme today). By the end of the model run, global ozone drops to less than 110 Dobson Units, a 67 percent drop from the 1970s.
To learn more about the experiment and what it taught scientists about stratospheric circulation, read the Earth Observatory Feature The World We Avoided by Protecting the Ozone Layer.
NASA images courtesy of the Goddard Space Flight Center Scientific Visualization Studio. Caption by Rebecca Lindsey.