

Carbon is the backbone of life on Earth. We are made of carbon, we eat carbon, and our civilizations—our economies, our homes, our means of transport—are built on carbon. We need carbon, but that need is also entwined with one of the most serious problems facing us today: global climate change.


Forged in the heart of aging stars, carbon is the fourth most abundant element in the Universe. Most of Earth’s carbon—about 65,500 billion metric tons—is stored in rocks. The rest is in the ocean, atmosphere, plants, soil, and fossil fuels.
Carbon flows between each reservoir in an exchange called the carbon cycle, which has slow and fast components. Any change in the cycle that shifts carbon out of one reservoir puts more carbon in the other reservoirs. Changes that put carbon gases into the atmosphere result in warmer temperatures on Earth.
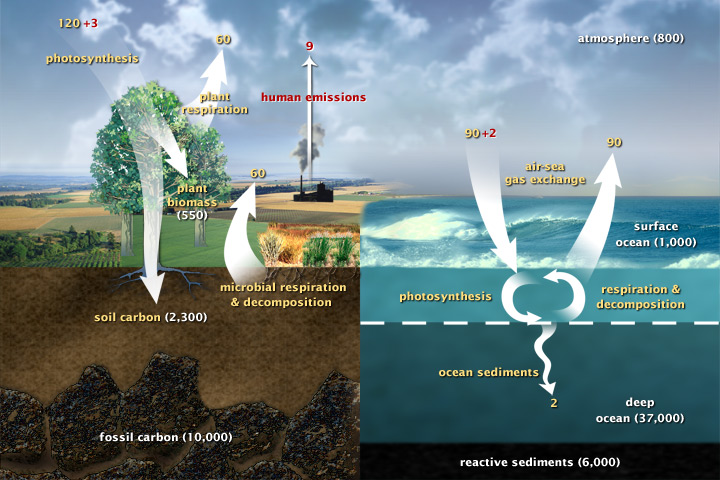
This diagram of the fast carbon cycle shows the movement of carbon between land, atmosphere, and oceans. Yellow numbers are natural fluxes, and red are human contributions in gigatons of carbon per year. White numbers indicate stored carbon. (Diagram adapted from U.S. DOE, Biological and Environmental Research Information System.)
Over the long term, the carbon cycle seems to maintain a balance that prevents all of Earth’s carbon from entering the atmosphere (as is the case on Venus) or from being stored entirely in rocks. This balance helps keep Earth’s temperature relatively stable, like a thermostat.
This thermostat works over a few hundred thousand years, as part of the slow carbon cycle. This means that for shorter time periods—tens to a hundred thousand years—the temperature of Earth can vary. And, in fact, Earth swings between ice ages and warmer interglacial periods on these time scales. Parts of the carbon cycle may even amplify these short-term temperature changes.
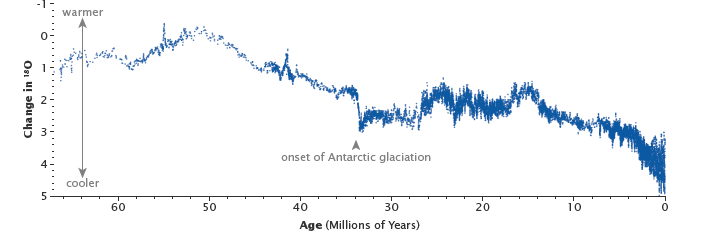
The uplift of the Himalaya, beginning 50 million years ago, reset Earth’s thermostat by providing a large source of fresh rock to pull more carbon into the slow carbon cycle through chemical weathering. The resulting drop in temperatures and the formation of ice sheets changed the ratio between heavy and light oxygen in the deep ocean, as shown in this graph. (Graph based on data from Zachos at al., 2001.)
On very long time scales (millions to tens of millions of years), the movement of tectonic plates and changes in the rate at which carbon seeps from the Earth’s interior may change the temperature on the thermostat. Earth has undergone such a change over the last 50 million years, from the extremely warm climates of the Cretaceous (roughly 145 to 65 million years ago) to the glacial climates of the Pleistocene (roughly 1.8 million to 11,500 years ago). [See Divisions of Geologic Time—Major Chronostratigraphic and Geochronologic Units for more information about geological eras.]
Through a series of chemical reactions and tectonic activity, carbon takes between 100-200 million years to move between rocks, soil, ocean, and atmosphere in the slow carbon cycle. On average, 1013 to 1014 grams (10–100 million metric tons) of carbon move through the slow carbon cycle every year. In comparison, human emissions of carbon to the atmosphere are on the order of 1015 grams, whereas the fast carbon cycle moves 1016 to 1017 grams of carbon per year.
The movement of carbon from the atmosphere to the lithosphere (rocks) begins with rain. Atmospheric carbon combines with water to form a weak acid—carbonic acid—that falls to the surface in rain. The acid dissolves rocks—a process called chemical weathering—and releases calcium, magnesium, potassium, or sodium ions. Rivers carry the ions to the ocean.

Rivers carry calcium ions—the result of chemical weathering of rocks—into the ocean, where they react with carbonate dissolved in the water. The product of that reaction, calcium carbonate, is then deposited onto the ocean floor, where it becomes limestone. (Photograph ©2009 Greg Carley.)
In the ocean, the calcium ions combine with bicarbonate ions to form calcium carbonate, the active ingredient in antacids and the chalky white substance that dries on your faucet if you live in an area with hard water. In the modern ocean, most of the calcium carbonate is made by shell-building (calcifying) organisms (such as corals) and plankton (like coccolithophores and foraminifera). After the organisms die, they sink to the seafloor. Over time, layers of shells and sediment are cemented together and turn to rock, storing the carbon in stone—limestone and its derivatives.

Limestone, or its metamorphic cousin, marble, is rock made primarily of calcium carbonate. These rock types are often formed from the bodies of marine plants and animals, and their shells and skeletons can be preserved as fossils. Carbon locked up in limestone can be stored for millions—or even hundreds of millions—of years. (Photograph ©2008 Rookuzz (Hmm).)
Only 80 percent of carbon-containing rock is currently made this way. The remaining 20 percent contain carbon from living things (organic carbon) that have been embedded in layers of mud. Heat and pressure compress the mud and carbon over millions of years, forming sedimentary rock such as shale. In special cases, when dead plant matter builds up faster than it can decay, layers of organic carbon become oil, coal, or natural gas instead of sedimentary rock like shale.

This coal seam in Scotland was originally a layer of sediment, rich in organic carbon. The sedimentary layer was eventually buried deep underground, and the heat and pressure transformed it into coal. Coal and other fossil fuels are a convenient source of energy, but when they are burned, the stored carbon is released into the atmosphere. This alters the balance of the carbon cycle, and is changing Earth’s climate. (Photograph ©2010 Sandchem.)
The slow cycle returns carbon to the atmosphere through volcanoes. Earth’s land and ocean surfaces sit on several moving crustal plates. When the plates collide, one sinks beneath the other, and the rock it carries melts under the extreme heat and pressure. The heated rock recombines into silicate minerals, releasing carbon dioxide.
When volcanoes erupt, they vent the gas to the atmosphere and cover the land with fresh silicate rock to begin the cycle again. At present, volcanoes emit between 130 and 380 million metric tons of carbon dioxide per year. For comparison, humans emit about 30 billion tons of carbon dioxide per year—100–300 times more than volcanoes—by burning fossil fuels.
Chemistry regulates this dance between ocean, land, and atmosphere. If carbon dioxide rises in the atmosphere because of an increase in volcanic activity, for example, temperatures rise, leading to more rain, which dissolves more rock, creating more ions that will eventually deposit more carbon on the ocean floor. It takes a few hundred thousand years to rebalance the slow carbon cycle through chemical weathering.

Carbon stored in rocks is naturally returned to the atmosphere by volcanoes. In this photograph, Russia’s Kizimen Volcano vents ash and volcanic gases in January 2011. Kizimen is located on the Kamchatka Peninsula, where the Pacific Plate is subducting beneath Asia. (Photograph ©2011 Artyom Bezotechestvo/Photo Kamchatka.)
However, the slow carbon cycle also contains a slightly faster component: the ocean. At the surface, where air meets water, carbon dioxide gas dissolves in and ventilates out of the ocean in a steady exchange with the atmosphere. Once in the ocean, carbon dioxide gas reacts with water molecules to release hydrogen, making the ocean more acidic. The hydrogen reacts with carbonate from rock weathering to produce bicarbonate ions.
Before the industrial age, the ocean vented carbon dioxide to the atmosphere in balance with the carbon the ocean received during rock weathering. However, since carbon concentrations in the atmosphere have increased, the ocean now takes more carbon from the atmosphere than it releases. Over millennia, the ocean will absorb up to 85 percent of the extra carbon people have put into the atmosphere by burning fossil fuels, but the process is slow because it is tied to the movement of water from the ocean’s surface to its depths.
In the meantime, winds, currents, and temperature control the rate at which the ocean takes carbon dioxide from the atmosphere. (See The Ocean’s Carbon Balance on the Earth Observatory.) It is likely that changes in ocean temperatures and currents helped remove carbon from and then restore carbon to the atmosphere over the few thousand years in which the ice ages began and ended.
The time it takes carbon to move through the fast carbon cycle is measured in a lifespan. The fast carbon cycle is largely the movement of carbon through life forms on Earth, or the biosphere. Between 1015 and 1017 grams (1,000 to 100,000 million metric tons) of carbon move through the fast carbon cycle every year.
Carbon plays an essential role in biology because of its ability to form many bonds—up to four per atom—in a seemingly endless variety of complex organic molecules. Many organic molecules contain carbon atoms that have formed strong bonds to other carbon atoms, combining into long chains and rings. Such carbon chains and rings are the basis of living cells. For instance, DNA is made of two intertwined molecules built around a carbon chain.
The bonds in the long carbon chains contain a lot of energy. When the chains break apart, the stored energy is released. This energy makes carbon molecules an excellent source of fuel for all living things.
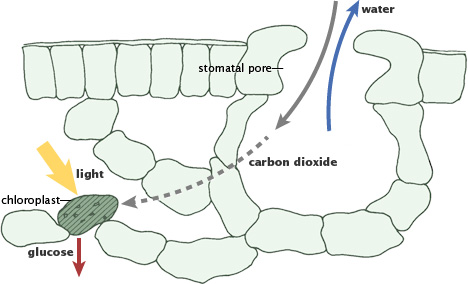
During photosynthesis, plants absorb carbon dioxide and sunlight to create fuel—glucose and other sugars—for building plant structures. This process forms the foundation of the fast (biological) carbon cycle. (Illustration adapted from P.J. Sellers et al., 1992.)
Plants and phytoplankton are the main components of the fast carbon cycle. Phytoplankton (microscopic organisms in the ocean) and plants take carbon dioxide from the atmosphere by absorbing it into their cells. Using energy from the Sun, both plants and plankton combine carbon dioxide (CO2) and water to form sugar (CH2O) and oxygen. The chemical reaction looks like this:
CO2 + H2O + energy = CH2O + O2
Four things can happen to move carbon from a plant and return it to the atmosphere, but all involve the same chemical reaction. Plants break down the sugar to get the energy they need to grow. Animals (including people) eat the plants or plankton, and break down the plant sugar to get energy. Plants and plankton die and decay (are eaten by bacteria) at the end of the growing season. Or fire consumes plants. In each case, oxygen combines with sugar to release water, carbon dioxide, and energy. The basic chemical reaction looks like this:
CH2O + O2 = CO2 + H2O + energy
In all four processes, the carbon dioxide released in the reaction usually ends up in the atmosphere. The fast carbon cycle is so tightly tied to plant life that the growing season can be seen by the way carbon dioxide fluctuates in the atmosphere. In the Northern Hemisphere winter, when few land plants are growing and many are decaying, atmospheric carbon dioxide concentrations climb. During the spring, when plants begin growing again, concentrations drop. It is as if the Earth is breathing.
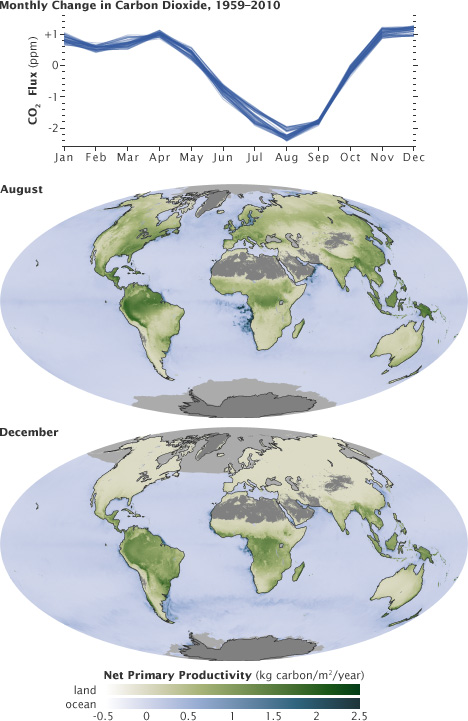
The ebb and flow of the fast carbon cycle is visible in the changing seasons. As the large land masses of Northern Hemisphere green in the spring and summer, they draw carbon out of the atmosphere. This graph shows the difference in carbon dioxide levels from the previous month, with the long-term trend removed.
This cycle peaks in August, with about 2 parts per million of carbon dioxide drawn out of the atmosphere. In the fall and winter, as vegetation dies back in the northern hemisphere, decomposition and respiration returns carbon dioxide to the atmosphere.
These maps show net primary productivity (the amount of carbon consumed by plants) on land (green) and in the oceans (blue) during August and December, 2010. In August, the green areas of North America, Europe, and Asia represent plants using carbon from the atmosphere to grow. In December, net primary productivity at high latitudes is negative, which outweighs the seasonal increase in vegetation in the southern hemisphere. As a result, the amount of carbon dioxide in the atmosphere increases.(Graph by Marit Jentoft-Nilsen and Robert Simmon, using data from the NOAA Earth System Research Laboratory. Maps by Robert Simmon and Reto Stöckli, using MODIS data.)
Left unperturbed, the fast and slow carbon cycles maintain a relatively steady concentration of carbon in the atmosphere, land, plants, and ocean. But when anything changes the amount of carbon in one reservoir, the effect ripples through the others.
In Earth’s past, the carbon cycle has changed in response to climate change. Variations in Earth’s orbit alter the amount of energy Earth receives from the Sun and leads to a cycle of ice ages and warm periods like Earth’s current climate. (See Milutin Milankovitch.) Ice ages developed when Northern Hemisphere summers cooled and ice built up on land, which in turn slowed the carbon cycle. Meanwhile, a number of factors including cooler temperatures and increased phytoplankton growth may have increased the amount of carbon the ocean took out of the atmosphere. The drop in atmospheric carbon caused additional cooling. Similarly, at the end of the last Ice Age, 10,000 years ago, carbon dioxide in the atmosphere rose dramatically as temperatures warmed.
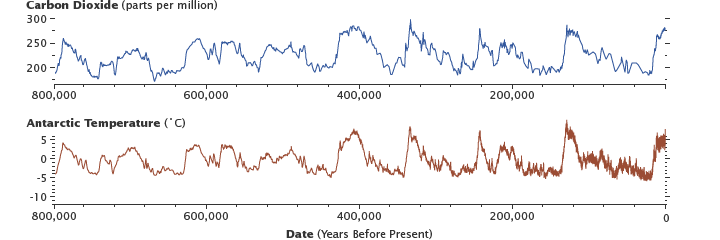
Levels of carbon dioxide in the atmosphere have corresponded closely with temperature over the past 800,000 years. Although the temperature changes were touched off by variations in Earth’s orbit, the increased global temperatures released CO2 into the atmosphere, which in turn warmed the Earth. Antarctic ice-core data show the long-term correlation until about 1900. (Graphs by Robert Simmon, using data from Lüthi et al., 2008, and Jouzel et al., 2007.)
Shifts in Earth’s orbit are happening constantly, in predictable cycles. In about 30,000 years, Earth’s orbit will have changed enough to reduce sunlight in the Northern Hemisphere to the levels that led to the last ice age.
Today, changes in the carbon cycle are happening because of people. We perturb the carbon cycle by burning fossil fuels and clearing land.
When we clear forests, we remove a dense growth of plants that had stored carbon in wood, stems, and leaves—biomass. By removing a forest, we eliminate plants that would otherwise take carbon out of the atmosphere as they grow. We tend to replace the dense growth with crops or pasture, which store less carbon. We also expose soil that vents carbon from decayed plant matter into the atmosphere. Humans are currently emitting just under a billion tons of carbon into the atmosphere per year through land use changes.

The burning of fossil fuels is the primary source of increased carbon dioxide in the atmosphere today. (Photograph ©2009 stevendepolo.)
Without human interference, the carbon in fossil fuels would leak slowly into the atmosphere through volcanic activity over millions of years in the slow carbon cycle. By burning coal, oil, and natural gas, we accelerate the process, releasing vast amounts of carbon (carbon that took millions of years to accumulate) into the atmosphere every year. By doing so, we move the carbon from the slow cycle to the fast cycle. In 2009, humans released about 8.4 billion tons of carbon into the atmosphere by burning fossil fuel.
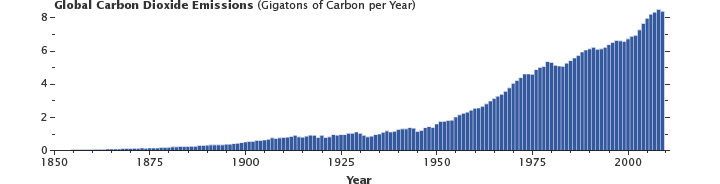
Emissions of carbon dioxide by humanity (primarily from the burning of fossil fuels, with a contribution from cement production) have been growing steadily since the onset of the industrial revolution. About half of these emissions are removed by the fast carbon cycle each year, the rest remain in the atmosphere. (Graph by Robert Simmon, using data from the Carbon Dioxide Information Analysis Center and Global Carbon Project.)
Since the beginning of the Industrial Revolution, when people first started burning fossil fuels, carbon dioxide concentrations in the atmosphere have risen from about 280 parts per million to 387 parts per million, a 39 percent increase. This means that for every million molecules in the atmosphere, 387 of them are now carbon dioxide—the highest concentration in two million years. Methane concentrations have risen from 715 parts per billion in 1750 to 1,774 parts per billion in 2005, the highest concentration in at least 650,000 years.
All of this extra carbon needs to go somewhere. So far, land plants and the ocean have taken up about 55 percent of the extra carbon people have put into the atmosphere while about 45 percent has stayed in the atmosphere. Eventually, the land and oceans will take up most of the extra carbon dioxide, but as much as 20 percent may remain in the atmosphere for many thousands of years.
The changes in the carbon cycle impact each reservoir. Excess carbon in the atmosphere warms the planet and helps plants on land grow more. Excess carbon in the ocean makes the water more acidic, putting marine life in danger.
It is significant that so much carbon dioxide stays in the atmosphere because CO2 is the most important gas for controlling Earth’s temperature. Carbon dioxide, methane, and halocarbons are greenhouse gases that absorb a wide range of energy—including infrared energy (heat) emitted by the Earth—and then re-emit it. The re-emitted energy travels out in all directions, but some returns to Earth, where it heats the surface. Without greenhouse gases, Earth would be a frozen -18 degrees Celsius (0 degrees Fahrenheit). With too many greenhouse gases, Earth would be like Venus, where the greenhouse atmosphere keeps temperatures around 400 degrees Celsius (750 Fahrenheit).

Rising concentrations of carbon dioxide are warming the atmosphere. The increased temperature results in higher evaporation rates and a wetter atmosphere, which leads to a vicious cycle of further warming. (Photograph ©2011 Patrick Wilken.)
Because scientists know which wavelengths of energy each greenhouse gas absorbs, and the concentration of the gases in the atmosphere, they can calculate how much each gas contributes to warming the planet. Carbon dioxide causes about 20 percent of Earth’s greenhouse effect; water vapor accounts for about 50 percent; and clouds account for 25 percent. The rest is caused by small particles (aerosols) and minor greenhouse gases like methane.
Water vapor concentrations in the air are controlled by Earth’s temperature. Warmer temperatures evaporate more water from the oceans, expand air masses, and lead to higher humidity. Cooling causes water vapor to condense and fall out as rain, sleet, or snow.
Carbon dioxide, on the other hand, remains a gas at a wider range of atmospheric temperatures than water. Carbon dioxide molecules provide the initial greenhouse heating needed to maintain water vapor concentrations. When carbon dioxide concentrations drop, Earth cools, some water vapor falls out of the atmosphere, and the greenhouse warming caused by water vapor drops. Likewise, when carbon dioxide concentrations rise, air temperatures go up, and more water vapor evaporates into the atmosphere—which then amplifies greenhouse heating.
So while carbon dioxide contributes less to the overall greenhouse effect than water vapor, scientists have found that carbon dioxide is the gas that sets the temperature. Carbon dioxide controls the amount of water vapor in the atmosphere and thus the size of the greenhouse effect.
Rising carbon dioxide concentrations are already causing the planet to heat up. At the same time that greenhouse gases have been increasing, average global temperatures have risen 0.8 degrees Celsius (1.4 degrees Fahrenheit) since 1880.
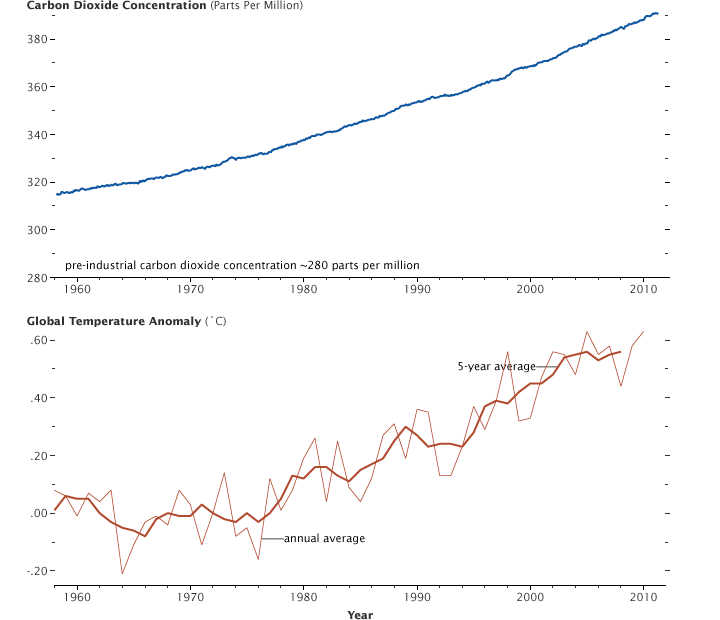
With the seasonal cycle removed, the atmospheric carbon dioxide concentration measured at Mauna Loa Volcano, Hawaii, shows a steady increase since 1957. At the same time global average temperatures are rising as a result of heat trapped by the additional CO2 and increased water vapor concentration. (Graphs by Robert Simmon, using CO2 data from the NOAA Earth System Research Laboratory and temperature data from the Goddard Institute for Space Studies.)
This rise in temperature isn’t all the warming we will see based on current carbon dioxide concentrations. Greenhouse warming doesn’t happen right away because the ocean soaks up heat. This means that Earth’s temperature will increase at least another 0.6 degrees Celsius (1 degree Fahrenheit) because of carbon dioxide already in the atmosphere. The degree to which temperatures go up beyond that depends in part on how much more carbon humans release into the atmosphere in the future.
About 30 percent of the carbon dioxide that people have put into the atmosphere has diffused into the ocean through the direct chemical exchange. Dissolving carbon dioxide in the ocean creates carbonic acid, which increases the acidity of the water. Or rather, a slightly alkaline ocean becomes a little less alkaline. Since 1750, the pH of the ocean’s surface has dropped by 0.1, a 30 percent change in acidity.

Some of the excess CO2 emitted by human activity dissolves in the ocean, becoming carbonic acid. Increases in carbon dioxide are not only leading to warmer oceans, but also to more acidic oceans. (Photograph ©2010 Way Out West News.)
Ocean acidification affects marine organisms in two ways. First, carbonic acid reacts with carbonate ions in the water to form bicarbonate. However, those same carbonate ions are what shell-building animals like coral need to create calcium carbonate shells. With less carbonate available, the animals need to expend more energy to build their shells. As a result, the shells end up being thinner and more fragile.
Second, the more acidic water is, the better it dissolves calcium carbonate. In the long run, this reaction will allow the ocean to soak up excess carbon dioxide because more acidic water will dissolve more rock, release more carbonate ions, and increase the ocean’s capacity to absorb carbon dioxide. In the meantime, though, more acidic water will dissolve the carbonate shells of marine organisms, making them pitted and weak.
Warmer oceans—a product of the greenhouse effect—could also decrease the abundance of phytoplankton, which grow better in cool, nutrient-rich waters. This could limit the ocean’s ability to take carbon from the atmosphere through the fast carbon cycle.
On the other hand, carbon dioxide is essential for plant and phytoplankton growth. An increase in carbon dioxide could increase growth by fertilizing those few species of phytoplankton and ocean plants (like sea grasses) that take carbon dioxide directly from the water. However, most species are not helped by the increased availability of carbon dioxide.
Plants on land have taken up approximately 25 percent of the carbon dioxide that humans have put into the atmosphere. The amount of carbon that plants take up varies greatly from year to year, but in general, the world’s plants have increased the amount of carbon dioxide they absorb since 1960. Only some of this increase occurred as a direct result of fossil fuel emissions.
With more atmospheric carbon dioxide available to convert to plant matter in photosynthesis, plants were able to grow more. This increased growth is referred to as carbon fertilization. Models predict that plants might grow anywhere from 12 to 76 percent more if atmospheric carbon dioxide is doubled, as long as nothing else, like water shortages, limits their growth. However, scientists don’t know how much carbon dioxide is increasing plant growth in the real world, because plants need more than carbon dioxide to grow.
Plants also need water, sunlight, and nutrients, especially nitrogen. If a plant doesn’t have one of these things, it won’t grow regardless of how abundant the other necessities are. There is a limit to how much carbon plants can take out of the atmosphere, and that limit varies from region to region. So far, it appears that carbon dioxide fertilization increases plant growth until the plant reaches a limit in the amount of water or nitrogen available.
Some of the changes in carbon absorption are the result of land use decisions. Agriculture has become much more intensive, so we can grow more food on less land. In high and mid-latitudes, abandoned farmland is reverting to forest, and these forests store much more carbon, both in wood and soil, than crops would. In many places, we prevent plant carbon from entering the atmosphere by extinguishing wildfires. This allows woody material (which stores carbon) to build up. All of these land use decisions are helping plants absorb human-released carbon in the Northern Hemisphere.

Changes in land cover—forests converted to fields and fields converted to forests—have a corresponding effect on the carbon cycle. In some Northern Hemisphere countries, many farms were abandoned in the early 20th century and the land reverted to forest. As a result, carbon was drawn out of the atmosphere and stored in trees on land. (Photograph ©2007 Husein Kadribegic.)
In the tropics, however, forests are being removed, often through fire, and this releases carbon dioxide. As of 2008, deforestation accounted for about 12 percent of all human carbon dioxide emissions.
The biggest changes in the land carbon cycle are likely to come because of climate change. Carbon dioxide increases temperatures, extending the growing season and increasing humidity. Both factors have led to some additional plant growth. However, warmer temperatures also stress plants. With a longer, warmer growing season, plants need more water to survive. Scientists are already seeing evidence that plants in the Northern Hemisphere slow their growth in the summer because of warm temperatures and water shortages.
Dry, water-stressed plants are also more susceptible to fire and insects when growing seasons become longer. In the far north, where an increase in temperature has the greatest impact, the forests have already started to burn more, releasing carbon from the plants and the soil into the atmosphere. Tropical forests may also be extremely susceptible to drying. With less water, tropical trees slow their growth and take up less carbon, or die and release their stored carbon to the atmosphere.
The warming caused by rising greenhouse gases may also “bake” the soil, accelerating the rate at which carbon seeps out in some places. This is of particular concern in the far north, where frozen soil—permafrost—is thawing. Permafrost contains rich deposits of carbon from plant matter that has accumulated for thousands of years because the cold slows decay. When the soil warms, the organic matter decays and carbon—in the form of methane and carbon dioxide—seeps into the atmosphere.
Current research estimates that permafrost in the Northern Hemisphere holds 1,672 billion tons (Petagrams) of organic carbon. If just 10 percent of this permafrost were to thaw, it could release enough extra carbon dioxide to the atmosphere to raise temperatures an additional 0.7 degrees Celsius (1.3 degrees Fahrenheit) by 2100.
Many of the questions scientists still need to answer about the carbon cycle revolve around how it is changing. The atmosphere now contains more carbon than at any time in at least two million years. Each reservoir of the cycle will change as this carbon makes its way through the cycle.
What will those changes look like? What will happen to plants as temperatures increase and climate changes? Will they remove more carbon from the atmosphere than they put back? Will they become less productive? How much extra carbon will melting permafrost put into the atmosphere, and how much will that amplify warming? Will ocean circulation or warming change the rate at which the ocean takes up carbon? Will ocean life become less productive? How much will the ocean acidify, and what effects will that have?
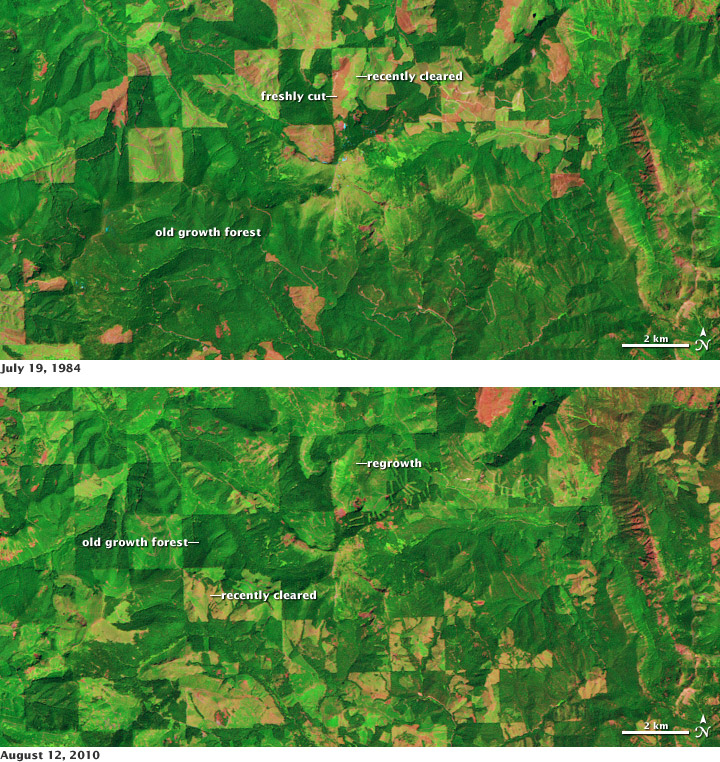
Time series of satellite data, like the imagery available from the Landsat satellites, allow scientists to monitor changes in forest cover. Deforestation can release carbon dioxide into the atmosphere, while forest regrowth removes CO2. This pair of false-color images shows clear cutting and forest regrowth between 1984 and 2010 in Washington State, northeast of Mount Rainier. Dark green corresponds to mature forests, red indicates bare ground or dead plant material (freshly cut areas), and light green indicates relatively new growth. (NASA image by Robert Simmon, using Landsat data from the USGS Global Visualization Viewer.)
NASA’s role in answering these questions is to provide global satellite observations and related field observations. As of early 2011, two types of satellite instruments were collecting information relevant to the carbon cycle.
The Moderate Resolution Imaging Spectroradiometer (MODIS) instruments, flying on NASA’s Terra and Aqua satellites, measure the amount of carbon plants and phytoplankton turn into matter as they grow, a measurement called net primary productivity. The MODIS sensors also measure how many fires occur and where they burn.
Two Landsat satellites provide a detailed view of ocean reefs, what is growing on land, and how land cover is changing. It is possible to see the growth of a city or a transformation from forest to farm. This information is crucial because land use accounts for one-third of all human carbon emissions.
Future NASA satellites will continue these observations, and also measure carbon dioxide and methane in the atmosphere and vegetation height and structure.
All of these measurements will help us see how the global carbon cycle is changing through time. They will help us gauge the impact we are having on the carbon cycle by releasing carbon into the atmosphere or finding ways to store it elsewhere. They will show us how our changing climate is altering the carbon cycle, and how the changing carbon cycle is altering our climate.
Most of us, however, will observe changes in the carbon cycle in a more personal way. For us, the carbon cycle is the food we eat, the electricity in our homes, the gas in our cars, and the weather over our heads. We are a part of the carbon cycle, and so our decisions about how we live ripple across the cycle. Likewise, changes in the carbon cycle will impact the way we live. As each of us come to understand our role in the carbon cycle, the knowledge empowers us to control our personal impact and to understand the changes we are seeing in the world around us.