

Kilauea is one of the world’s most active volcanoes, but it is of the sort that tends to ooze lava more often than it explodes. Until March 19, 2008, the last explosive eruption from the Halema`uma`u Crater, the summit crater, occurred in 1924. But starting on March 19, a small explosion from the crater rained rock and ash over the summit. The explosion heralded further activity at the summit, including a two- to four-fold increase in the amount of sulfur dioxide seeping from the volcano. The Hawaiian Volcano Observatory warned on March 28 that sulfur dioxide concentrations in the air downwind from the volcano were likely to be hazardous, particularly to children or those with asthma or other breathing difficulties. Even before the March 19 explosion, elevated sulfur dioxide levels prompted the National Park Service to close part of Crater Rim Drive starting in mid-February.
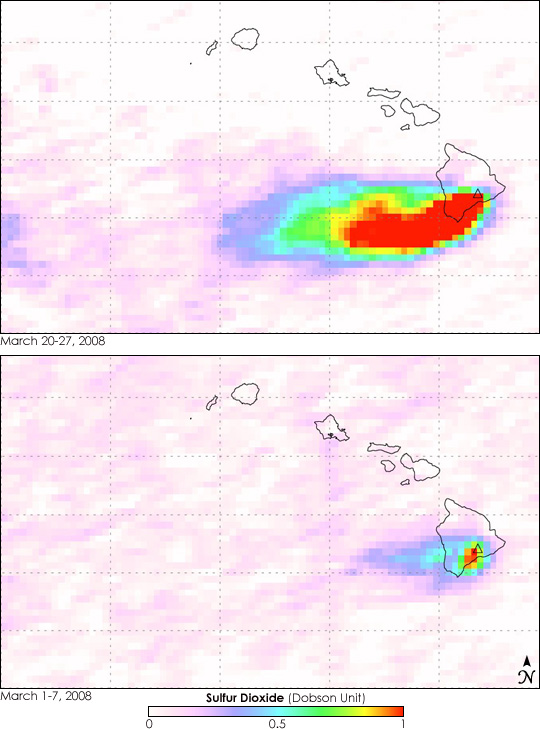
The Ozone Monitoring Instrument (OMI) on NASA’s Aura satellite recorded the increase in sulfur dioxide rising out of Kilauea between March 20 and March 27, 2008, top. Throughout the period, the easterly trade winds swept a long plume of sulfur dioxide south and west, away from major populated areas. The highest concentrations of the gas are shown in red. The lower image shows more typical sulfur dioxide levels as observed between March 1 and March 7, 2008. This plume is much smaller and contains significantly less sulfur dioxide.
OMI measures sulfur dioxide in Dobson Units. If you could compress all the sulfur dioxide in a column of the atmosphere into a flat layer at the Earth’s surface (at 0 degrees Celsius), one Dobson Unit of the gas would be 0.01 millimeters thick, and it would contain 0.0285 grams of sulfur dioxide per square meter.
In addition to its potential public health impacts, sulfur dioxide can also affect climate. During explosive eruptions, volcanic gases and particles may reach altitudes of more than 12 miles (20 kilometers). Sulfur dioxide gas reacts with water in the atmosphere to make sulfate aerosol particles. These light-colored particles can linger in the stratosphere for months following a big eruption, reflecting sunlight and cooling Earth.
NASA image courtesy Simon Carn, Joint Center for Earth Systems Technology (JCET), University of Maryland Baltimore County (UMBC). The OMI instrument is a Dutch-Finnish Instrument, provided to the EOS/Aura mission by The Netherlands and Finland. Caption by Holli Riebeek, with information from Simon Carn.